The FDA launched their up to date steering on Cybersecurity in medical gadgets: High quality System Issues and content material for Premarket submissions on the finish of June in 2025. On this steering the FDA emphasizes the expectation that cybersecurity dangers and vulnerabilities be addressed in a risk mannequin. Cybersecurity procedures to help merchandise from preliminary design to finish of life have to be carried out. Sufficient cybersecurity protections have to be addressed within the design inputs throughout growth and never “bolted on” after growth is accomplished.
If you’re a developer or producer that’s or will probably be liable for a linked medical system, the cybersecurity course of is one thing you have to make investments effort in now.
There are a number of requirements comparable to ANSI/AAMI SW96:2023 and IEC 81001-5-1: 2021 which are well known within the US and within the EU that element what actions and deliverables must be addressed relative to cybersecurity danger evaluation for medical system software program. This brief article is targeted on learn how to carry out the evaluation which is the core a part of the cybersecurity risk mannequin. The target for this text is to supply perception on conducting safety danger evaluation that’s impactful for the entire lifetime of a medical system or medical system software program.
Gadget cybersecurity planning
From a pre-market perspective, the important thing components to a safety evaluation are as follows:
 Determine 1 – Cybersecurity steps in danger evaluation
Determine 1 – Cybersecurity steps in danger evaluation
Along with planning for cybersecurity medical system corporations now must implement a selected framework of their high quality system. To catch up to a degree of compliance some corporations are making the error of addressing compliance by investing in a one-tool-does-all software program choice, however this may increasingly probably miss the true wants to handle cybersecurity in your group. The wholistic resolution is an software of a Safe Product Improvement Framework that addressed the actions and duties all through the whole product lifecycle (TPLC). Commissioning sources to develop and replace processes and SOP’s is required however the core of getting a strong course of is to ascertain an efficient and environment friendly danger evaluation methodology. The chance evaluation methodology is represented because the Safety Danger Evaluation within the evaluation part of determine 1.
Deal with Property in Safety Evaluation Desk
The Safety Danger Evaluation is used to determine potential safety danger controls and determine vulnerabilities. Two tables must be established, a Danger Evaluation desk that identifies and maintains the safety danger controls and the continuing vulnerabilities desk monitoring the present vulnerabilities. The 2 evaluation tables might be in separate tabs on a easy spreadsheet as they’ve filtering and sorting capabilities to permit for long run administration of the tables. The content material for the Danger Evaluation Desk is mirrored in Desk 1 addressing the Safety Controls.
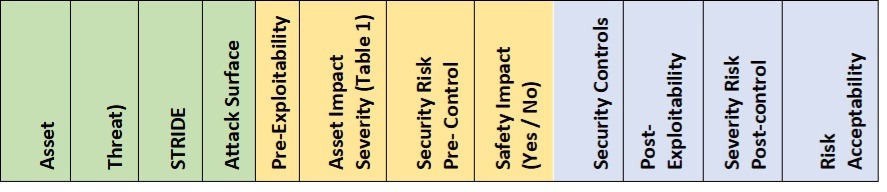
Desk 1 – Safety Danger Evaluation evaluation desk
The main target of the Safety Danger Evaluation must be the belongings outlined within the safety structure and the way threats could exploit vulnerabilities to compromise the belongings.
The belongings are components which are of worth to the consumer, affected person, consumer group and enterprise which are related to safety. Property may typically embrace:
- Knowledge dealt with and saved on the product (e.g., Logs, buyer or operational information, configuration information)
- Knowledge of private nature (e.g., Protected Well being Info/PHI)
- Credential data (e.g., passwords, credentials, keys)
- Software program/firmware of the product
- Third get together companies (e.g., cloud companies, open supply libraries, API’s).
The evaluation ought to embrace fairly sensible threats to every of the belongings. The STRIDE mannequin might be utilized to determine the potential risk in every evaluation row. The requirements STRIDE mannequin, and the specified safety property is offered in Desk 2 under.
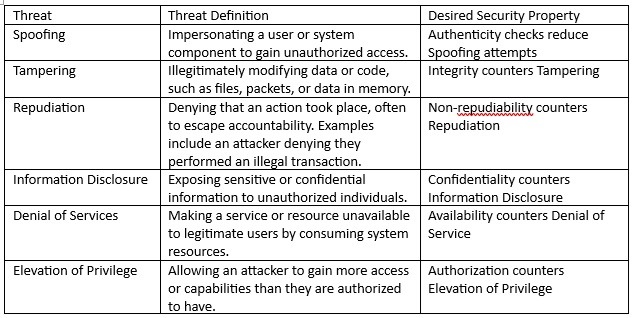
Desk 2 – Stride definitions
Vulnerability identification
Along with the Safety Danger Evaluation the separate ongoing vulnerabilities desk must be maintained, as talked about beforehand. This desk accommodates the identified vulnerabilities which were recognized within the system. Vulnerabilities are flaws or weaknesses that might be exploited by the potential threat-sources. Vulnerabilities might be recognized by means of:
- Figuring out vulnerabilities within the Safety Danger Evaluation
- cybersecurity penetration evaluation and testing of the product
- by means of static evaluation scanning of internally developed software program with SAST (Static Utility Safety Testing) instrument
- by means of a dynamic evaluation scanning with DAST (Dynamic Utility Safety Testing) instrument
- reviewing present catalogs just like the Frequent Vulnerabilities and Publicity Checklist, the Nationwide Vulnerability Database (NVD), the Nationwide Well being ISAC, and launch notes of SOUP/COTS.
- recognized by means of vulnerability scanning utilizing a SCA (Software program Composition Evaluation) instrument for OTS or open-source elements.
The analysis of identified vulnerabilities is important previous to any product launch and maintained till the product is retired. As vulnerabilities are recognized and addressed, they could be faraway from the continuing vulnerabilities evaluation desk. Actions on the vulnerabilities are pushed by a severity willpower of the vulnerability and the CVSS rating. The CVSS scoring mannequin identifies six base objects in its matrix for every of the recognized vulnerabilities: Assault Vector (AV), Assault Complexity (AC), Privileges Required (PR), Consumer Interplay (UI), Scope (S) and Impression (CIA) measuring confidentiality, integrity and availability loss.
Decide affect of threats
In security danger evaluation we all know that danger is the mixture of severity and likelihood of hurt (Danger = P x S). Nevertheless, for safety danger evaluation the FDA recommends contemplating the exploitability of a possible vulnerability versus the usage of likelihood of an assault occurring with success. Exploitability must be based mostly on how susceptible a system is to being compromised. The higher the safety controls the decrease the chance. The acceptability analysis of threats to belongings and vulnerabilities must be pushed by a desk matrix like to at least one in Desk 3, pushed by severity of hurt and exploitability.
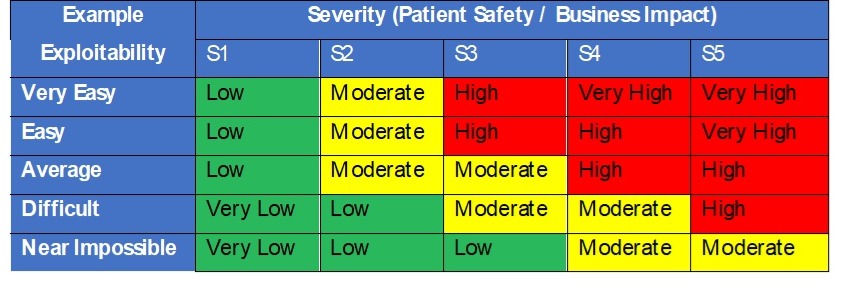
Desk 3 – Safety exploitability and severity choice desk
Larger dangers would require documented danger controls within the Safety Danger Evaluation desk to scale back their exploitability and total danger. Danger controls ought to then drive necessities within the design and are subsequently verified as efficient throughout design verification.
Conclusion
The safety evaluation of dangers and vulnerabilities must be maintained in two tables, as one is required to determine and preserve the safety dangers and the opposite is an ongoing record of vulnerabilities that require periodic updates after launch. These two evaluation tables are to be up to date periodically and the continuing vulnerability record drive patch and buyer notification throughout product upkeep.
Whether or not your organization could also be simply beginning out or at the next level within the cybersecurity maturity spectrum of reaching a sturdy and environment friendly cybersecurity framework, a deal with the format and evaluation core practices as a precedence and can result in higher choices on instruments and enhancements within the course of effectivity and competency.
Picture: Traitov, Getty Photographs
Bob Barrett, vice chairman of techniques engineering at Full Spectrum, is an skilled engineering chief and group builder with a deal with outcomes. He has technical strengths gained from greater than 30 years in medical system techniques engineering, techniques and software program validation, security danger evaluation, high quality administration and mission administration. Bob spent 15 years with Baxter’s drug supply division, the place he led the techniques engineering group. Bob has the function of participant coach at Full Spectrum, main the techniques engineering group, whereas offering his deep insights on to shoppers. Bob is a robust believer in cadence pushed mission administration methods. His capacity to be hands-on or lead cross-functional groups accelerates shopper’s medical growth applications from idea to market launch.
This put up seems by means of the MedCity Influencers program. Anybody can publish their perspective on enterprise and innovation in healthcare on MedCity Information by means of MedCity Influencers. Click here to find out how.



